Osmosis is the net movement of water across a selectively permeable membrane driven by a difference in solute concentrations on the two sides of the membrane. Describe the relationship.
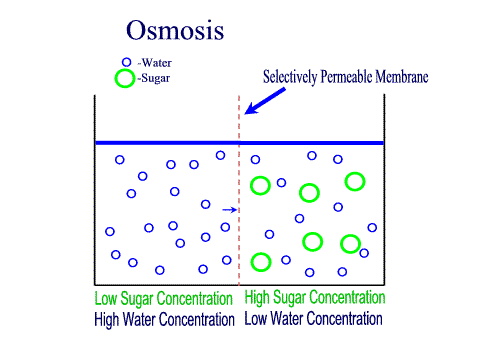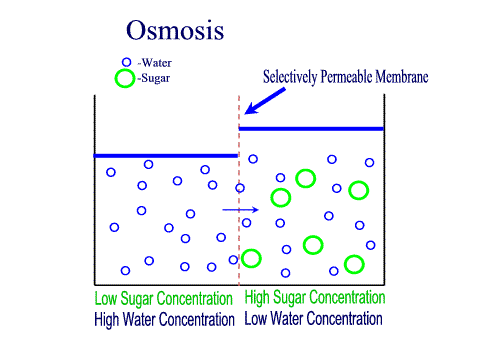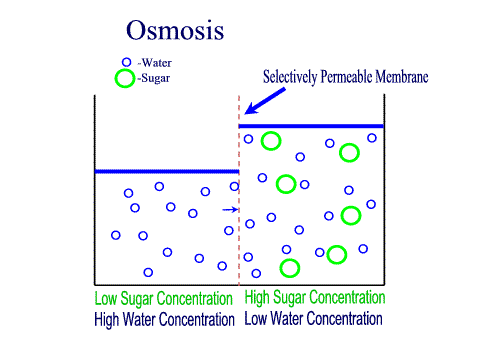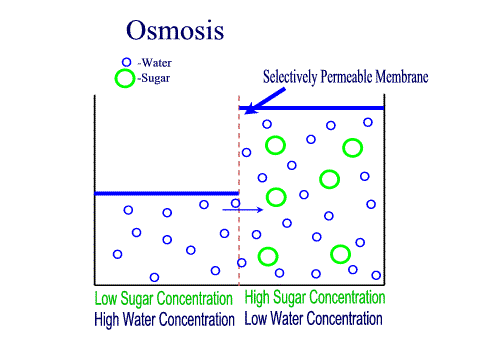
What Is The Definition Of Osmosis In Terms Of Solute Concentration Socratic
You cannot differentiate one substance from another within the solution.
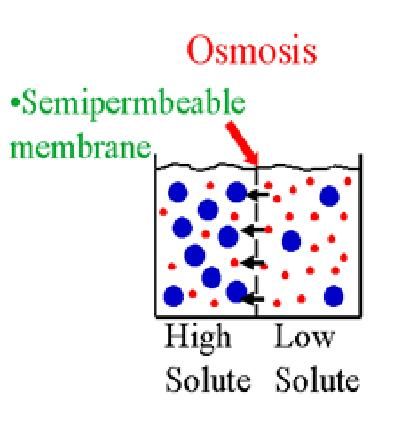
. An Easy and Ugly Explanation. A 1 Sucrose solution or a 50 Sucrose solution. According to the Oxford dictionary of the English language osmosis is a natural process in which solvent molecules such as water tend to pass through a semipermeable membrane membrane that only allow the passage of the solvent molecules 2 into a region of greater solute concentration in order to establish a balanced concentration of the solvent 1.
Important factors to Osmosis and Diffusion include Temperature Concentration and. The substance in which the solute is dissolved is called a solvent. Solute Solvent Solution.
When one substance dissolves into another a solution is formedà A solution is a homogeneous mixture of a solute dissolved in a solventà The solute is the substance that dissolves while the solvent is the dissolving mediumà Solutions can be formed with different types of. 100 1 rating Osmosis is the phenomenon of movement of solvent across a semipermeable membrane towards a higher concentration of solute lower concentration of solvent from lower concentration of solute higher concentration of solvent. Diffusion of solvent through semi-permeable membrane into a region of higher solute concentration in the direction that tends to.
Osmosis and Net Movement of Water. The maximum amount of a solute that will dissolve in a particular solvent at a specific temperature and pressure Diffusion The movement of molecules from an area of high concentration to an area of low concentration high to low. In any situation the more concentrated a solution is in terms of solute the less concentrated it is in terms of solvent.
A solvent is usually a liquid. This is important in osmosis as you have to be careful which way round. The process by which one thing absorbs or is absorbed by another.
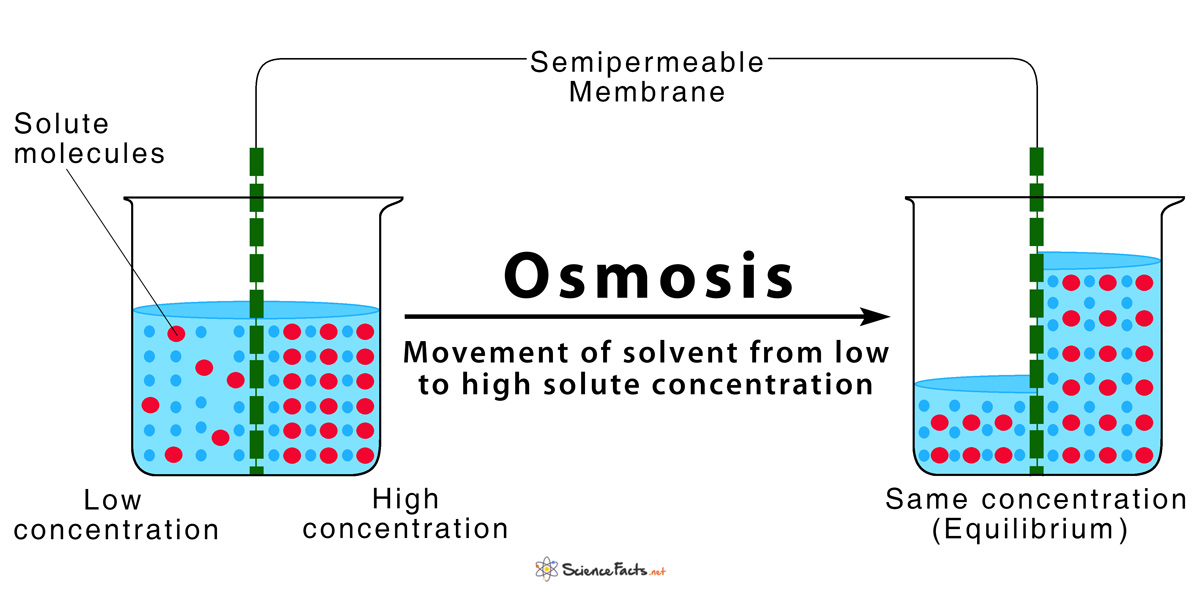
The net movement of solvent molecules usually water from a region of lower solute concentration to a region of higher solute concentration through a partially permeable membrane. Osmosis ɒ z ˈ m oʊ s ɪ s US also ɒ s- is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane from a region of high water potential region of lower solute concentration to a region of low water potential region of higher solute concentration in the direction that tends to equalize the solute concentrations on the. Start studying Bio Chapter 4.
1 2 3 osmosis. Movement of substances across cell membrane without need for energy input. Did the water move into or out of all three model.
Other times it makes it harder to remember what youre studying. The dispersed step of a solution is known as the solute. Solute solvent diffusion semi-permeable membrane.
Learn vocabulary terms and more with flashcards games and other study tools. A solute is a substance that is added to a solvent to form a solution. Look at your osmosis data in Table 1.
Osmosis is a process in which a solvent difuses from a region ofhigh concentration of solute to a region of low concentration ofsolute across a semi-permeable membrane. The solute is the dispersed phase of a solution. Osmosis is the spontaneous net movement of solvent molecules through a partially permeable membrane into a region of higher solute concentration in the direction that tends to equalize the solute concentrations on the two sides.
Diffusion of molecules through a semipermeable membrane. The solvent is the medium phase of a solution that disperses solute particles. The part of a solution that is present in the greatest amount is called a solvent.
This is one of those times that alliteration makes things harder. Sometimes alliteration consecutive words that all start with the same sound can be helpful. Which solution has the highest concentration of water the solvent.
Diffusion or osmosis occurs until dynamic equilibrium has been reached. Determine the solute and solvent for the solution outside the cell environment and for the inside of the cell. The substance which is dissolved is called a solute.
Main characteristics of a solution Solution is homogenous. We review their content and use your feedback to keep the quality high. Do the words hypertonic hypotonic and isotonic refer to the solute or solvent concentration.
What about when it comes to solute solvent and solution. That the solution is a homogenous mixture means that it forms a single phase. The solvent is the solutions medium step which disperses the solute particles.
Osmosis is a special kind of diffusion where water moves through a selectively permeable membrane that only allows certain molecules to diffuse though Lab Manual 7e 2010. Osmosis is similar to Diffusion but its the process in which water moves across a semi-permeable membrane and goes to the higher concentration of solute1 22. Osmosis Worksheet.
A solvent is a substance that dissolves the solute particles during the formation of a solution. Use the following words in your answer. A selectively permiable membrane is one that allows unrestricted passage of water but not solute molecules or ions.
In a solution the amount of solute is less than the amount of solvent. Its the liquid that the solute is dissolved in. Give the direction of the net movement of water into cell out of cell or into out of cell at equal rates.

How Does Osmosis Relate To Solute Concentration Socratic

0 Comments